Abstract
Introduction: Splenic Marginal Zone Lymphoma (SMZL) is an uncommon subtype of non-Hodgkin Lymphoma, therefore, the data concerning the diagnosis, prognosis and treatment are scare and mostly based on a limited number of retrospective series. In 2014, GELTAMO group launched a guidelines for practical management of SMZL to be used in the everyday practice in this nation-wide network of collaborative hospitals. (GEL-LZME-2014-14). These Guidelines proposed/suggested different treatment strategies according to the HPLLs/ABC score (Montalban et al, 2012). Therapeutic recommendations were observation (W&W) in group A, rituximab in group B and rituximab in combination with chemotherapy (R-Chemotherapy) in group C. The primary endpoint was to analyze compliance with the guidelines and to evaluate the outcome according to the HPLLs/ABC-adapted therapeutic strategy in the real life.
Methods: Observational multicenter study of patients with diagnostic of SMZL between 2014 and 2019 included prospectively and consecutively in the RELINF registry by Spanish GELTAMO centers. Clinical data at diagnosis, HPLLs/ABC prognostic index, treatment, response and follow up were obtained from the medical records and entered into a database. Lymphoma Specific Survival (LSS) and response rates according to treatment received were assessed in this study. Composite event free survival (CEFS) defined as death, histological transformation (HT), recurrence/progression under treatment, and need for treatment under observation, was also analyzed. HT was considered as a time-dependent event.
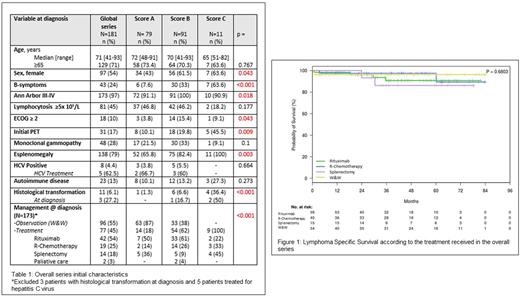
Results: A total of 181 patients (84M/97F; median age 71 years) from 22 centers were included. The main initial characteristics of the patients are shown in Table 1. Splenectomy was performed in 17 cases (9%). 57% of the patients followed the recommendation of the GELTAMO Guidelines. 13 cases were excluded for response and survival analyses (i.e., 3 with HT at diagnosis, 5 treated for hepatitis C virus and 5 managed with palliative care). At diagnosis 77 patients (45%) received some therapy while 96 (55%) remained on W&W (Table 1). The overall response rate was higher in the R-Chemotherapy arm and in the Rituximab arm compared to splenectomy (p<0.001), with no significant differences between Rituximab and R-Chemotherapy. With a median follow up of 41.2 months, the 5-year overall survival (OS) of the whole series was 77%, and the 5-year LSS of 93%, with 32 patients (18%) dying, but only 13 (40%) due to the lymphoma or its treatment. 5-year LSS was 98% and 87% for score A and B, respectively (p=0.048), but unexpectedly, there were no deaths among the 9 patients of score C (5-year LSS: 100%). There were no differences in the 5-year LSS according to the treatment received in the overall series (p=0.68) (Figure 1), nor when scores A and B were analyzed separately. The 5-year CEFS in the overall series was 46%. Analyzing the composed event according to score, there were significant differences in terms of the 5-year CEFS between scores A and B (p=0.044). When comparing LSS and progression-free survival (PFS) in patients treated with Rituximab or R-Chemotherapy at diagnosis or after the W&W period, there were no significant differences between the two groups. Patients on score C had a higher risk of HT than the remaining groups (HT at 5 years: 1.3%, 6.6% and 36% for scores A, B and C, respectively; p<0.001). HT was associated with significantly lower OS (Cox-time dependent: HR 7.816, 95% CI 3.179-19.217; p<0.001).
Conclusions: Herein we describe the everyday practice for diagnosis, stratification, and treatment of SMZL in Spain. Overall, 57% of the patients were managed according to the Guidelines. Our data support that Rituximab is the best treatment, when needed. Patients with score A who do not need immediate treatment after diagnosis and are followed with W&W policy have a very good outcome. In patients with scores A and B who were observed after initial diagnosis, the response to Rituximab at the time of need of therapy was not hampered when compared to patients receiving Rituximab immediately after diagnosis. HT is associated with reduced survival. The low number of cases in score C prevents us from drawing conclusions in this group at this time.
Disclosures
Villalobos:Janssen: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau. Muntañola Prat:Janssen: Honoraria, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau. González de Villambrosia:Takeda: Honoraria; Janssen: Honoraria; Incyte: Honoraria; EusaPharma: Honoraria. Cordoba:Kite: Consultancy; GenMab: Consultancy; Takeda: Consultancy; Celgene: Consultancy, Honoraria; Bristol Myers: Research Funding; Pfizer: Consultancy, Speakers Bureau; Gilead: Honoraria. Bastos-Oreiro:JANSSEN: Speakers Bureau; INCYTE: Consultancy, Speakers Bureau; NOVARTIS: Speakers Bureau; KITE/GILEAD: Consultancy, Honoraria; Roche: Consultancy, Research Funding, Speakers Bureau. Sancho:Bristol Myers Squibb: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biomedicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eli Lilly & Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kern Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. García:Janssen, Roche, Gilead, Celgene, Abbvie: Other: medical meetings funding; Janssen, Abbvie: Research Funding; Janssen, Roche, Gilead, Celgene: Consultancy. Abrisqueta:AbbVie: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy; Sandoz: Honoraria; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau. Terol:Janssen, Abbvie, Roche, Takeda, Astra-Zeneca: Consultancy. López-Guillermo:Roche: Research Funding; Roche, Kite/Gilead, Celgene, Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Hospital Clinic de Barcelona: Current Employment. Salar:Incyte: Speakers Bureau; roche: Research Funding, Speakers Bureau; Gilead: Research Funding; Janssen: Honoraria, Speakers Bureau; BMS: Honoraria; Abbvie: Research Funding; Beigene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal